
HEY THERE! THIS BLOG IS DEDICATED TO SCHOOLWORK, AS WELL AS ACADEMIC ADVICE (THROUGH PERSONAL EXPERIENCE) AND SOME CRAFTING/HOBBIES. I HOPE YOU'LL FIND MY BLOG TO BE USEFUL AND ENTERTAINING!
Featured Posts
(T1B2) Crafting Fun
This summer I was very interested in photo editing and using ebay to sell my crafts. I found this really useful book that went over al...

Monday, October 31, 2016
Sunday, October 30, 2016
(T1B44) Halloween! Jack-O-Lantern
I can't wait untill Halloween! I'm going with some of my friends to trick or treat around Kerrsidale! Later on we're going to eat pizza and watch a scary movie. It's going to be so awesome! By the way I'm going to be a devil!

Wednesday, October 26, 2016
(T1B35) Chemistry
My science fair project has a lot of chemistry... I have to understand this:
"The active ingredient in the Indicator Solution is Sulphide anions (S–). When mixed with water, reacts with lead cations (Pb++) to produce a distinctive brownish-black color. The chemical equation is as follows:
Na2S + H2O + Pb++ > PbS + Na+ + OH- + H2S ~
Sodium Sulphide mixed with water and lead produces lead sulphide (PbS). Bi- products in reaction include hydrogen sulfide H2S gas, sodium ions (Na+) and hydroxide ions (OH-)
Balanced equation: 2Na2S + 2H2O + Pb++ > PbS + 4Na+ + 2OH- + H2S"
"The active ingredient in the Indicator Solution is Sulphide anions (S–). When mixed with water, reacts with lead cations (Pb++) to produce a distinctive brownish-black color. The chemical equation is as follows:
Na2S + H2O + Pb++ > PbS + Na+ + OH- + H2S ~
Sodium Sulphide mixed with water and lead produces lead sulphide (PbS). Bi- products in reaction include hydrogen sulfide H2S gas, sodium ions (Na+) and hydroxide ions (OH-)
Balanced equation: 2Na2S + 2H2O + Pb++ > PbS + 4Na+ + 2OH- + H2S"
I'm pretty sure that I'm screwed.
Friday, October 21, 2016
(T1B34) The Evil Mirror
What happens when a toddler is secretly the head of the Evil coroporation behind your back.
(T1B31) Emailing
I just spent my whole day emailing etsy artists for donations for the NPO project. I sent like 5 pages worth of messages (roughly equivalent to about 90 messages?). I'm determined to do well for this project.
Thursday, October 20, 2016
(T1B29) Unique!
I am so happy right now! I just finished developing a completely unique project for my science fair that has great implementations in the real world! Also my npo project is going GREAT! We just got 3 offers to donate mugs to our company, so we can begin selling really soon!
Sunday, October 16, 2016
(T1B28) Carbon Cloth
I don't know where I should buy carbon cloth for my electrodes in the mfc! Some are so expensive (like $20 for a 2 x 2 cm piece). I don't know the surface area for the other one though... Here is the link for the cloth I want to buy: eBay Link.

Thursday, October 13, 2016
(T1B27) Science Fair Proposals
Hey guys! I'm going to be writing a series of blogs about my science fair proposals.
1. Construct a mfc as previously described. Soil samples will be collected 5 cm below ground, and will be immediatly transferred to the anode after dampening with water. The cell will be incubated at 25 - 30 degrees celcius. Multimeter readings will be taken after a stable current is acheived.
Notes: a few innovations will be applied; in the anode, a tube will be attached to a bottle to deliver substrates by gravitational drip. The tube will be placed next to the biofilm, or where the electrode base is. Another tube will be attached to a container filled with water to remove excess gas from the anode. The whol mfc will be placed on top of a large plastic container to catch any possible leaks.
Methods to reduce internal resistance: reduce distance between electrodes, increase ion concentrations in salt bridge (increase conductivity). Considerations to use graphite rods for electrodes will be investigated.
Part II will be in the next blog!
The proposals are due next class :(.
1. Construct a mfc as previously described. Soil samples will be collected 5 cm below ground, and will be immediatly transferred to the anode after dampening with water. The cell will be incubated at 25 - 30 degrees celcius. Multimeter readings will be taken after a stable current is acheived.
Notes: a few innovations will be applied; in the anode, a tube will be attached to a bottle to deliver substrates by gravitational drip. The tube will be placed next to the biofilm, or where the electrode base is. Another tube will be attached to a container filled with water to remove excess gas from the anode. The whol mfc will be placed on top of a large plastic container to catch any possible leaks.
Methods to reduce internal resistance: reduce distance between electrodes, increase ion concentrations in salt bridge (increase conductivity). Considerations to use graphite rods for electrodes will be investigated.
Part II will be in the next blog!
The proposals are due next class :(.
Wednesday, October 12, 2016
(T1B26) Hurricane?
I recently read about a potential storm and three hurricanes that are coming to metro Vancouver and some other places tonight... I'm pretty worried about it. It's supposed to be a level 4 hurricane.


I don't get why they look so pretty from space though!
Tuesday, October 11, 2016
(T1B25) Chemistry :(
There's a lot of chemistry in my science project that's really hard :(.
"The element has 82 protons and 82 electrons for a net neutral charge. There are 5 electron shells that are completely filled and 4 electrons in the 6th shell. The outer 4 electrons are available for chemical reactions. (Refer to the shell model which shows a dot for each electron). These outer electrons are named 6s2 and 6p2 and they can escape from the atom through a chemical reaction called oxidation to create a lead ion that has a +2 charge (missing 2 electrons) or a +4 charge (missing 4 electrons). The molecule that receives the electrons from Pb will be reduced. Oxidation and reduction reactions always occur together."
I'll need to do a lot of readings later.

"The element has 82 protons and 82 electrons for a net neutral charge. There are 5 electron shells that are completely filled and 4 electrons in the 6th shell. The outer 4 electrons are available for chemical reactions. (Refer to the shell model which shows a dot for each electron). These outer electrons are named 6s2 and 6p2 and they can escape from the atom through a chemical reaction called oxidation to create a lead ion that has a +2 charge (missing 2 electrons) or a +4 charge (missing 4 electrons). The molecule that receives the electrons from Pb will be reduced. Oxidation and reduction reactions always occur together."
I'll need to do a lot of readings later.
(T1B24) Volunteer Opportunities
So I've been reflecting on possible volunteer opportunites for a while... Here's a list I compiled:
- Food Bank - cooking such as heating soup or slicing bread
- Blood Donations - give cookies to people who just donated blood
- Heart and Stroke - knock on doors and ask for donations
Eh. It'll probably be a while until I secure a position. Hopefully I can do research at the blood research thing sometime soon.
Saturday, October 8, 2016
(T1B23) Mechanisms of Bio remediation?
I'm sort of puzzled behind the mechanisms of bio remediation from the bacteria living in the fuel cells. Many research papers revealed that the main mechanism of heavy metal reduction is adsorption. I was hoping that they could also metabolize the bacteria into less toxic forms.

(T1B22) Samples?
I'll be getting my soil samples to run my microbial fuel cells near the Fraser river and perhaps the heavy metal contaminated gardens? I'm really hoping that the samples contain a lot of living anaerobic bacteria. If not it would take forever for them to grow into a bio film.

Here are the principles involved with soil based microbial fuel cells. Of course I doing a lot of changes to its construction so it generate more power.
Here are the principles involved with soil based microbial fuel cells. Of course I doing a lot of changes to its construction so it generate more power.
(T1B21) Decisions
Right now I'm not sure what I should do... I could either go on with the project idea I have right now or make it more complex so that I can go to Safoni Biogenius Challenge. I know that if I do choose to go to the Biogenius Challenge, I would probably not do so well at CWSF or regionals... But I would be the youngest there.
(T1B20) Microbial Fuel Cells!
I decided to work on bio remediation in microbial fuel cells. This way we can generate electricity while treating waste! Here is a diagram on how a fuel cell works:
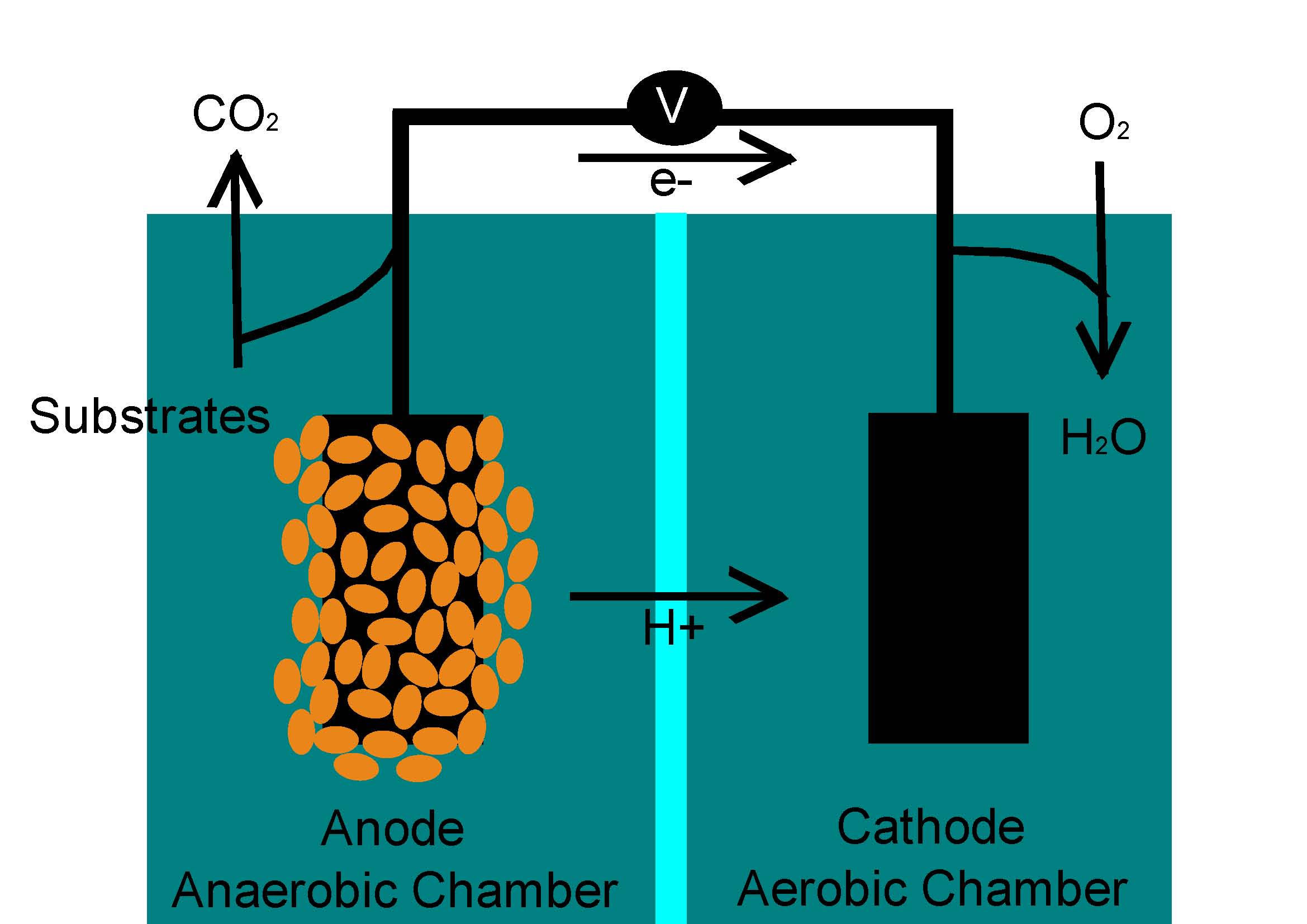

I'm actually pretty excited to start my project. I have been reading quite a lot of research papers so I can get some background information on my topic, and so far everything's going well...
(T1B19) Progress
Ok so the science fair proposal is due in about a week... I did massive amounts of research these days (that's why I'm so exhausted). Anyways I narrowed down my idea. It's not going to be about antibiotic resistance anymore so I'm working on a renewable energy project.
Sunday, October 2, 2016
(T1B18) Mentors...
Birthday!
My friend got me a fragrance mist from Bath and Body works, as well as this pretty black notebook! I had a nice dinner and cake at home and my dad got me a Wacom pen for ipad air 2! I can't believe he would actually buy such an expensive pen... (It's like 99 bucks).
My friend got me a fragrance mist from Bath and Body works, as well as this pretty black notebook! I had a nice dinner and cake at home and my dad got me a Wacom pen for ipad air 2! I can't believe he would actually buy such an expensive pen... (It's like 99 bucks).
| This is the pretty fragrance mist my friend got me!!! |
(T1B17) Mentors...
Hey!
I started writing my emails for possible mentors from the UBC microbiology/biochemistry department. Unfortunately none of the professors actually do research on bacteriophages, so I may need to change topics eventually... So far I sent out a email to Natalie Strynadka, a biochemist specializing in antibiotic resistance. I'm pretty sure she won't respond though.
I started writing my emails for possible mentors from the UBC microbiology/biochemistry department. Unfortunately none of the professors actually do research on bacteriophages, so I may need to change topics eventually... So far I sent out a email to Natalie Strynadka, a biochemist specializing in antibiotic resistance. I'm pretty sure she won't respond though.
| Their labs look AMAZING though! |
Subscribe to:
Comments (Atom)




